Carbon is the basic building block of all life, and is even found in the ocean, the air, and rocks. The organic carbon in all land-based organisms (alive and dead) is equal to 500 gigatons. The total organic and inorganic carbon in the top layer of the crust - the pedosphere - equals 1500 gigatons!
When carbon attaches itself to oxygen in the atmosphere it creates CO2, commonly known as carbon dioxide. Carbon dioxide is a greenhouse gas that locks in heat. Without carbon dioxide in our atmosphere, Earth would be a frozen planet. However, there is more carbon dioxide in the atmosphere than there used to be (30% more than there was 150 years ago), which has increased the Earth’s temperature.
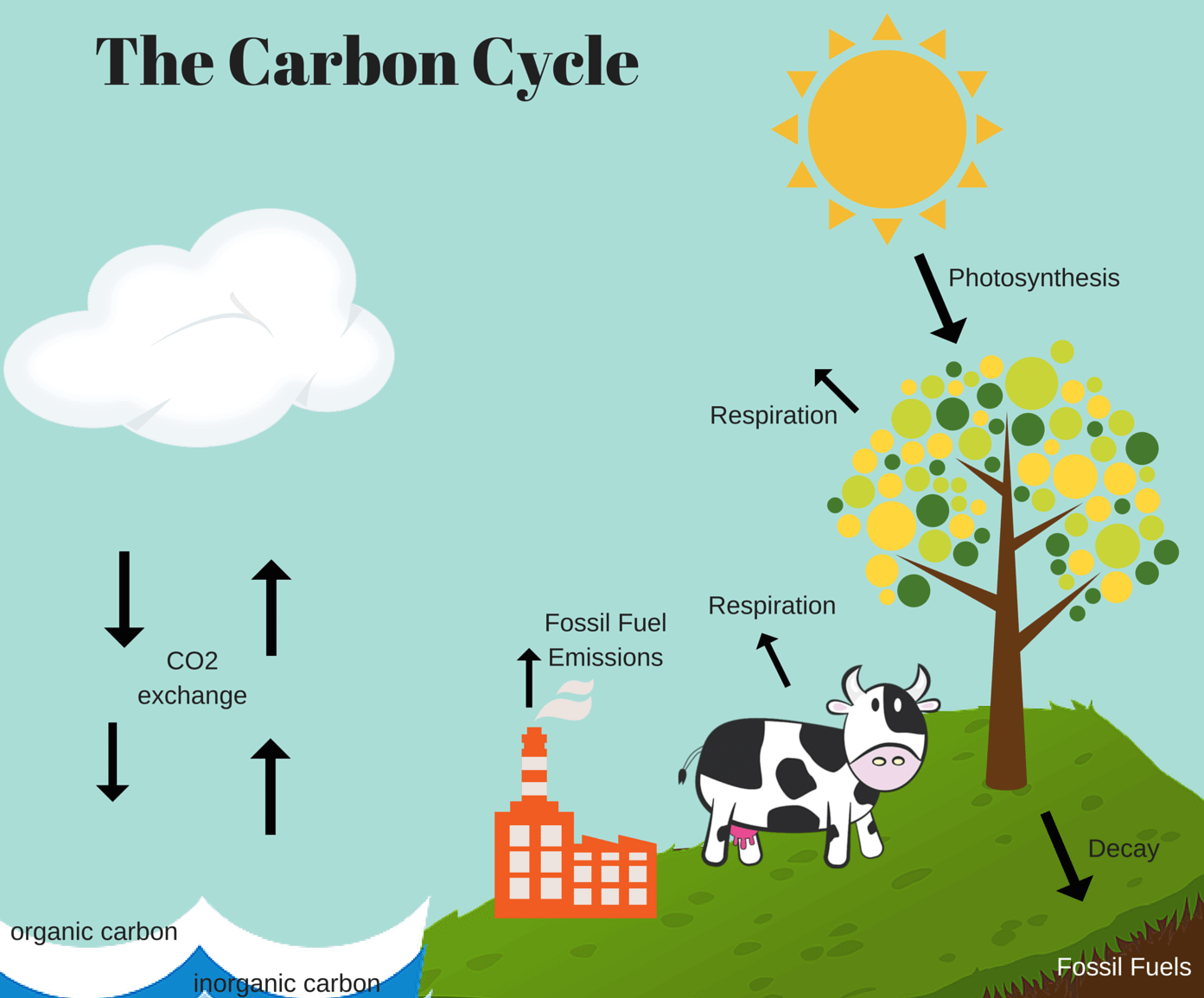
Carbon is not only found in all living things (and some non-living), it also has a biogeochemical cycle, passing through the biosphere - global sum of all ecosystems -, lithosphere - Earth’s crust and upper mantle -, hydrosphere - combined mass of water -, and the atmosphere - layer of gases around the Earth. This cycle is known as the carbon cycle.
Within the carbon cycle, autotrophs (plants, algae, and some bacteria) use carbon dioxide and sunlight, in the process known as photosynthesis, to grow and flourish. During this process carbon becomes a part of the autotroph until it is destroyed. When trees take carbon dioxide in, it is not released until the wood is burned or rotted; cutting down a tree will not release carbon dioxide.
When an autotroph dies, the elements of it, including carbon dioxide, are absorbed by the lithosphere and may turn into fossil fuels over millions of years. Fossil fuels are extracted from the ground by humans and used in a variety of ways, releasing the carbon dioxide back into the atmosphere.
In contrast, heterotrophs (humans, animals, fungi, and some bacteria) ingest carbon compounds by eating other organisms. Carbon dioxide is produced through aerobic respiration - a process which requires oxygen.
Carbon dioxide also leaves the atmosphere by dissolving into a body of water, forming carbonic acid, a chemical compound that contributes to overall ocean acidity. The Earth’s oceans have the greatest quantity of actively cycled carbon. The oceans’ surface layers contain a large amount of organic carbon that is constantly being exchanged with the atmosphere. Organic carbon can come from the atmosphere as carbonic acid or it can come as dissolved organic carbon from a river system. Oceanic organisms use photosynthesis to process the carbon. The dead tissue from these organisms sinks to deeper ocean levels, where a larger amount of carbon, known as inorganic carbon, is stored for longer periods of time before becoming sediment or returning to the surface layers.
The Earth’s lithosphere plays a special role in the carbon cycle. When carbon is bound in the lithosphere it is naturally taken out of the cycle until it is manually extracted and burned. Most of the carbon in the lithosphere is found in limestone - a sedimentary rock composed of calcite and aragonite. Limestone is often formed from the sedimentation of calcium carbonate found in the decayed shells of marine organisms. A smaller portion of carbon in the lithosphere is composed of kerogens - mixtures of organic chemical compounds that are a part of the organic matter in sedimentary rocks. Kerogens are made from the sedimentation and burial of terrestrial organisms under high heat and pressure. Carbon dioxide can be released from the lithosphere through volcanoes, volcanic regions, or the extraction and burning of fossil fuels. When the rate of carbon extraction from the lithosphere exceeds the rate that carbon is fixed, extra carbon dioxide enters the atmosphere.
Carbon runs through its cycle continuously either through respiration, burning, rotting, and photosynthesis. But as fossil fuels are extracted and burned the total amount of carbon dioxide in the system increases, which raises the Earth’s overall temperature. Higher amounts of carbon dioxide also raise the temperature and acidity of the oceans. This limits the ocean’s ability to absorb carbon and reduces oceanic biodiversity.
